baeyer test|Baeyer's Test : Bacolod Uses of Baeyer’s Reagent: Test for Unsaturation: Detects alkenes and alkynes by causing a color change from pinkish-purple to brown, indicating the presence . 3D Swertres results on Thursday, 6 June, 2019 from PCSO in the Philippines. Latest Results. Home; About; Blog; Contact; Login; Thursday, 6 June 2019 3D Swertres Results PCSO by GIDApp. . Updated: Wed 01/12/2022 17:12 GMT+8 : 3D Swertres Results 2019-06-05 . 3D Swertres Results .
PH0 · Tests for Unsaturation
PH1 · Baeyer’s Reagent
PH2 · Baeyers Test
PH3 · Baeyer's Test
PH4 · Baeyer's Reagent: Test, Preparation, Properties
PH5 · Baeyer test
PH6 · BAEYER'S TEST
PH7 · 8: Identification of Unknowns (Experiment)
PH8 · 6.4D: Individual Tests
Engineering is a very broad discipline, but among the various disciplines, which are the top 10 hardest engineering courses in the World? You will find out shortly. Studying engineering is no joke, it is considered to be one of the most difficult courses in the world - because it requires a good knowledge of mathematics
baeyer test*******Learn how to perform the baeyers test to detect unsaturated carbon carbon bonds, such as alkenes or alkynes, using potassium permanganate solution. Follow the procedure, .Learn how to identify unsaturated hydrocarbons using alkaline potassium permanganate (Baeyer's test) and bromine test. Find out the theory, .
Permanganate (Baeyer) Test. A potassium permanganate \(\left( \ce{KMnO_4} \right)\) solution is a test for unsaturation (alkenes and alkynes) or functional groups that can be .
Uses of Baeyer’s Reagent: Test for Unsaturation: Detects alkenes and alkynes by causing a color change from pinkish-purple to brown, indicating the presence .
baeyer testLearn how to use Baeyer's reagent, an alkaline solution of potassium permanganate, to detect unsaturation in organic compounds. Find out the mechanism, examples, and FAQs of this test.A test for unsaturated compounds in which potassium permanganate is used. Alkenes, for example, are oxidised to glycols, and the permanganate loses its colour:3R 2 C=CR 2 .Permanganate Test for Unsaturation (Baeyer Test): Aqueous permanganate rapidly oxidizes double and triple bonds while being reduced to MnO2, a brown precipitate. .
163. 8.9K views 3 years ago #GOC #Organicchemistry #organictest. Baeyer’s reagent is an alkaline solution of cold potassium permanganate (KMnO4). It is a useful oxidizing agent and used in the. #Baeyerstest #Synaddition #POCThis video explains the Beyer's test which used to identify the unsaturation in any compound with examples.
Baeyer's Test The synthesis of indigo starting from o-nitrocinnamic acid, due to Baeyer, was adapted byHoppe-Seyler as test for glucose in urine. Sinc e important steps in this route a re missing, as well as . 2. Alkaline KMnO, Test (Baeyer’s Test) In this test the pink colour potassium permanganate disappears when an alkaline potassium permanganate is added to an unsaturated hydrocarbon. The disappearance of pink colour may take place with or without the formation of brown precipitate of manganese oxide. ProcedureLa prueba de Baeyer se usa para encontrar enlaces carbono-carbono insaturados, como en el caso de los alquenos o alquinos, pero no para enlaces carbono-carbono aromáticos. La solución de permanganato de potasio (KMnO4) es de color morado. Cuando el permanganato de potasio reacciona con un alqueno, la solución pasa de ser morada a .
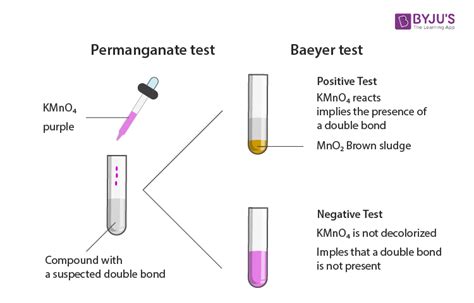
Although the tests work well in general, when using a chemical test to support identification of a structure, caution should be used in interpretation of the results. For example, aldehydes are stated to give a positive result in the bromine test, which is when the compound turns the orange bromine solution clear.
Although the tests work well in general, when using a chemical test to support identification of a structure, caution should be used in interpretation of the results. For example, aldehydes are stated to give a positive result in the bromine test, which is when the compound turns the orange bromine solution clear. Permanganate (Baeyer) Test. A potassium permanganate \(\left( \ce{KMnO_4} \right)\) solution is a test for unsaturation (alkenes and alkynes) or functional groups that can be oxidized (aldehydes and some alcohols, Figure 6.66). The permanganate ion \(\left( \ce{MnO_4^-} \right)\) is a deep purple color, and upon .KMnO 4 is used in qualitative organic analysis to test for the presence of unsaturation. It is sometimes referred to as Baeyer's reagent after the German organic chemist Adolf von Baeyer. The reagent is an alkaline solution of potassium permanganate. Reaction with double or triple bonds (-C=C- or -C≡C-) causes the color to fade from purplish .
Baeyer test Source: A Dictionary of Chemistry Author(s): John DaintithJohn Daintith. A test for unsaturated compounds in which potassium permanganate is used. Alkenes, for example, are oxidised to glycols, and the .This test is also known as the Baeyer test. Here, potassium permanganate, KMnO 4, is used as an oxidizing agent to convert the alkene or alkyne to a diol (two alcohol functional groups in the same molecule). The visual colour change is from dark purple (permanganate solution) to dark green (manganate solution) then to black precipitate .
Learn it through the basic concepts explained here.#chemistry #chemicalreactions #learnchemistry #funchemistry #learningmadeeasyB. Potassium Permanganate (the Baeyer Test) A second qualitative test for unsaturation, the Baeyer test, depends on the ability of potassium permanganate to oxidize the carbon‑carbon double bond to give alkanediols or the carbon-carbon triple bond to give carboxylic acids .
The Baeyer test is used less frequently than the bromination test to determine if a compound is an alkene because the Baeyer solution can react with other functional groups on the molecule as well as any alkene groups and be decolorized. The following reaction equation shows how ethene molecules can be reacted with cold, dilute, alkaline .
Baeyer’s reagent is an alkaline solution of cold potassium permanganate (KMnO4). It is a useful oxidizing agent and used in the qualitative organic analysis. To test an organic compound for unsaturation using Bayer's test. Although the tests work well in general, when using a chemical test to support identification of a structure, caution should be used in interpretation of the results. For example, aldehydes are stated to give a positive result in the bromine test, which is when the compound turns the orange bromine solution clear. Baeyer test. A test for unsaturated compounds in which potassium permanganate is used. Alkenes, for example, are oxidised to glycols, and the permanganate loses its colour: 3R 2 C=CR 2 + 2KMnO 4 + 4H2O → 2MnO 2 + 2KOH + 3R 2 COHR 2 COH. Posted 17th November 2018 by Akram Amir El Ali.baeyer test Baeyer's Test There are two tests that we can conduct for alkenes on unsaturation. They are: Baeyer's test. Bromine solution test. In Baeyer's test, alkenes will react with the potassium permanganate which is an alkaline solution. And thus the colour of potassium permanganate will slowly fade as it forms glycol on the chemical reaction.Die Baeyer-Probe, benannt nach Adolf von Baeyer, ist ein qualitatives Verfahren der analytischen Chemie, um ungesättigte Kohlenwasserstoffverbindungen, . Phenol- und Anilinderivate sowie Mercaptane und Thioether einen positiven Test. Dabei erfolgt die Oxidation von Alkinen mit Kaliumpermanganat unübersichtlich. This test is much more selective for alkenes than Baeyer's Test and therefore a better way to confirm your compound has a double bond. Lucas Test. The first of the many tests for alcohol is the Lucas test, whereby you add zinc chloride and concentrated hydrochloric acid to your compound. If it contains an alcohol attached to a .
CroxyProxy is a free proxy to unblock Youtube, Facebook, Google and other favorite websites. Avoid network blockages at home, office or school. The best choice from any proxy list over the Internet.
baeyer test|Baeyer's Test